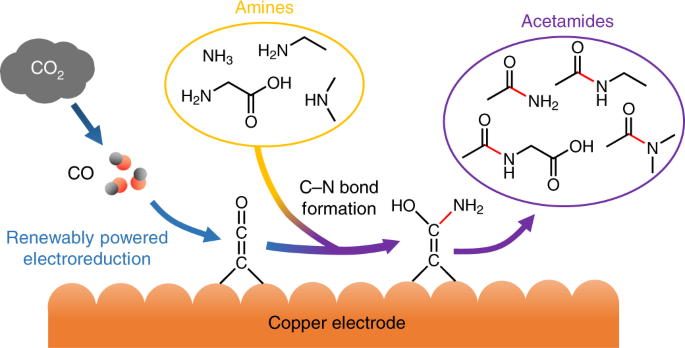
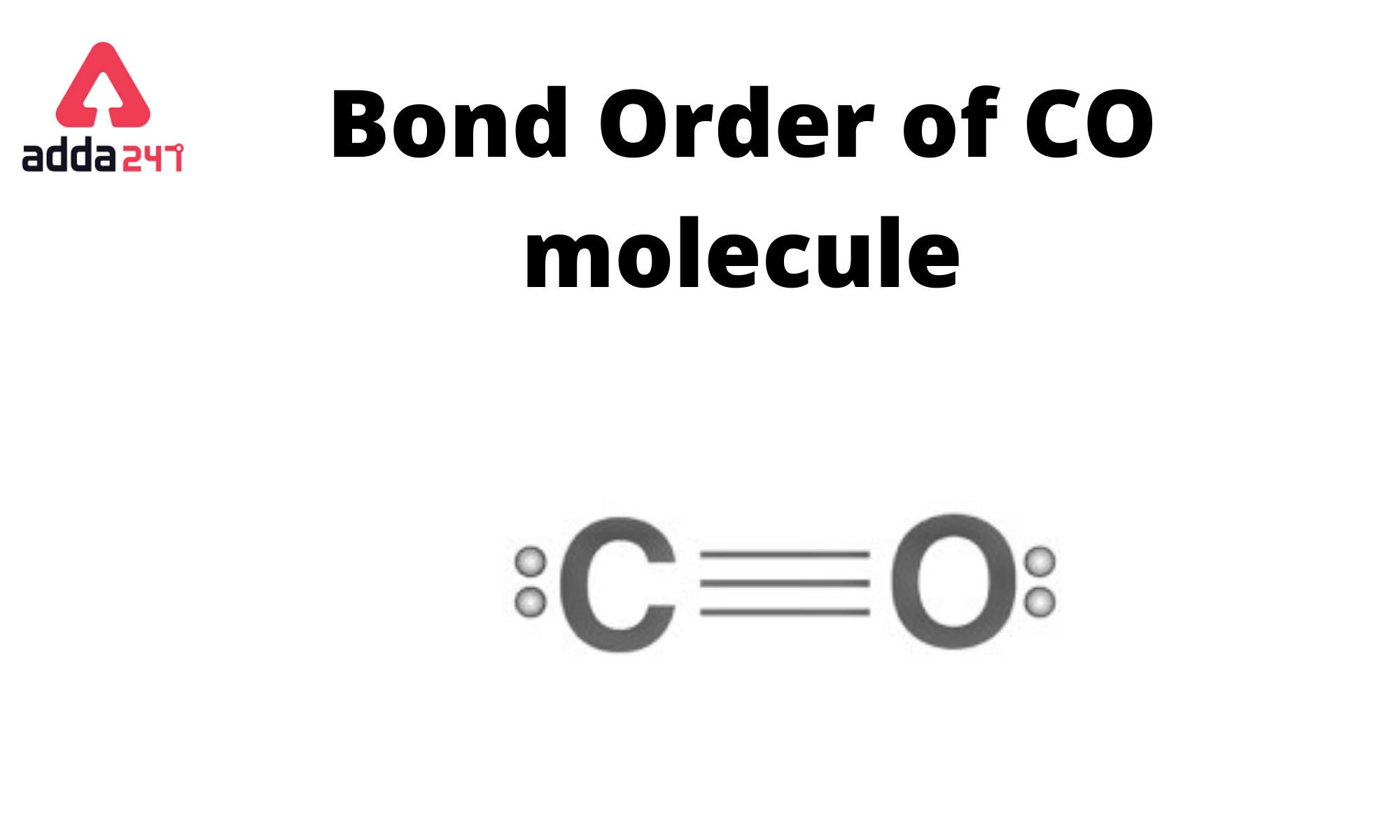
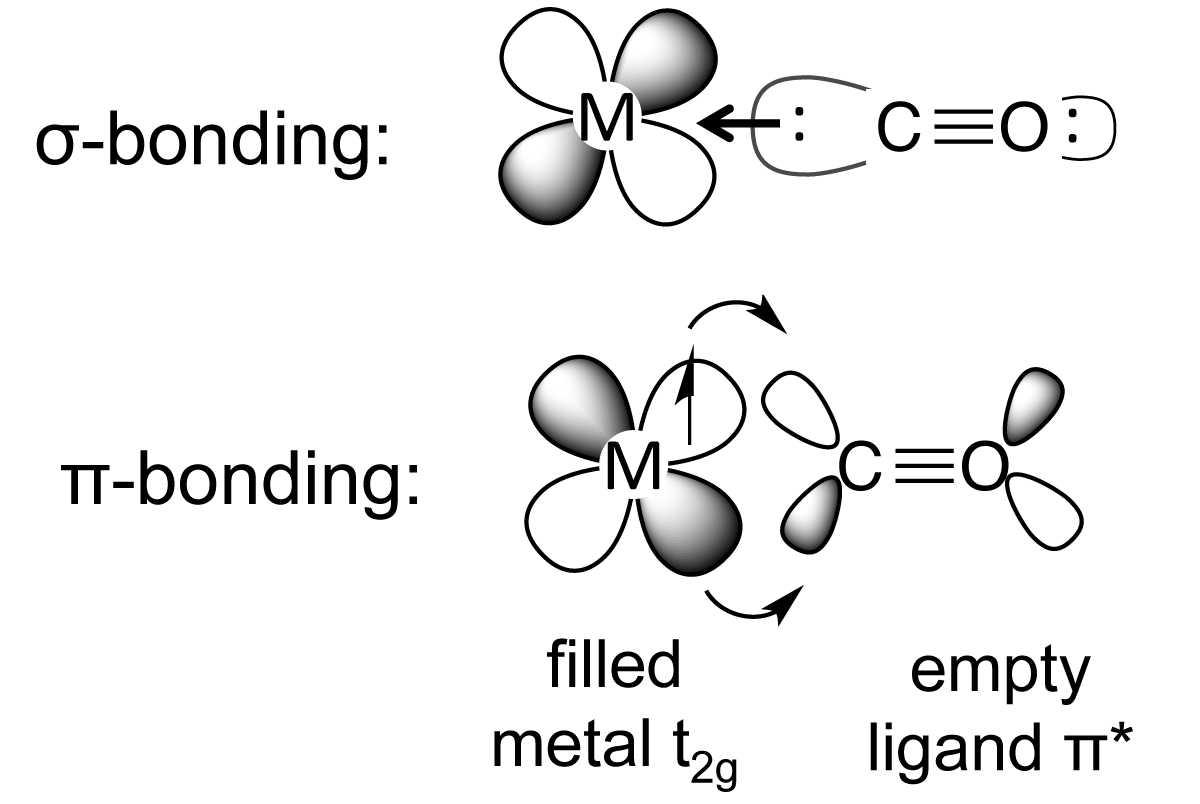
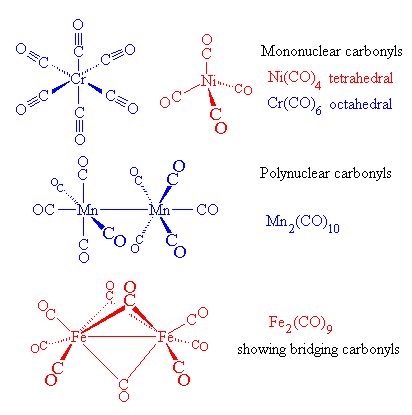
orbitals - How to rationalise with MO theory that CO is a two-electron donor through carbon? - Chemistry Stack Exchange
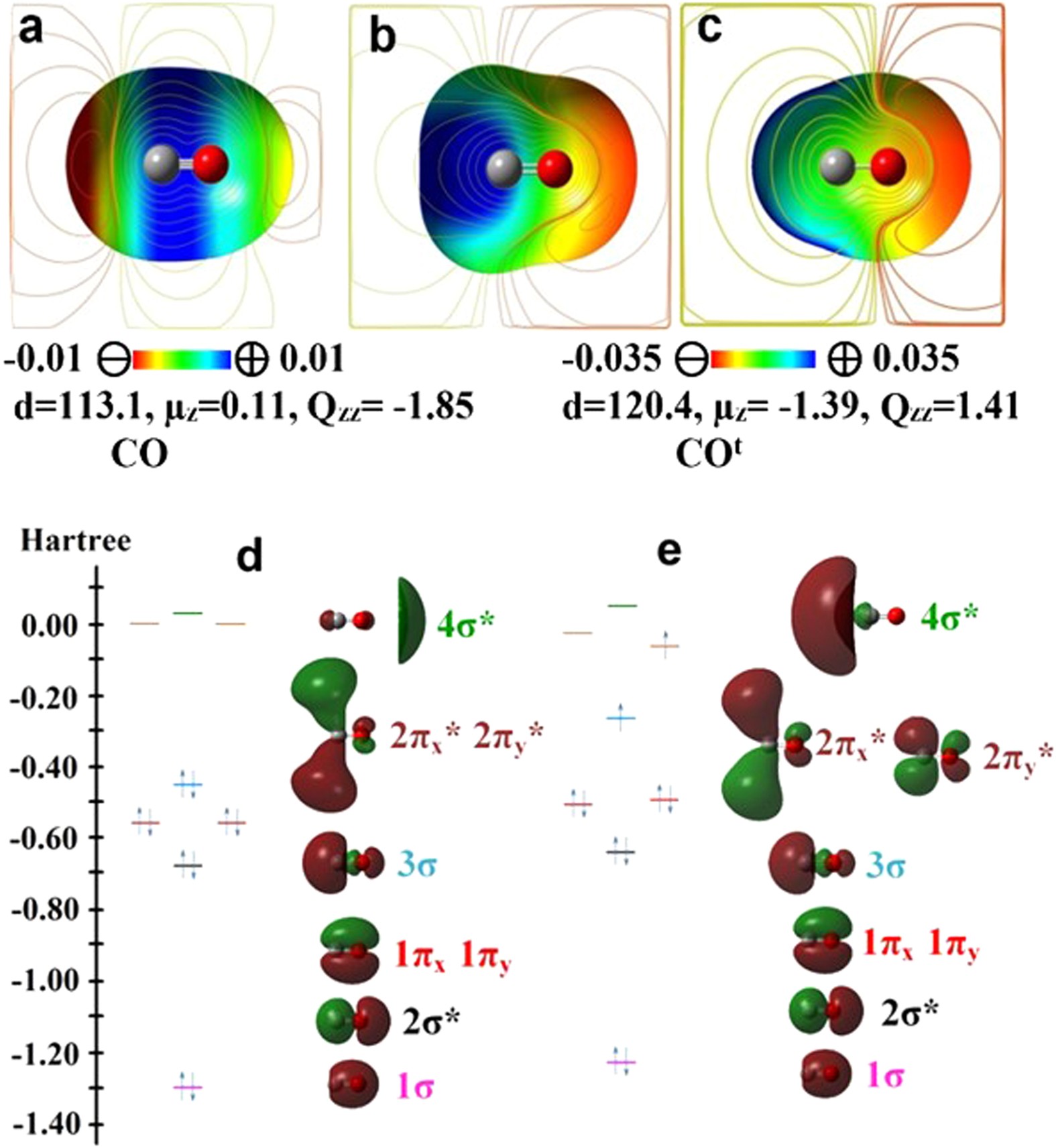
Intriguing Electrostatic Potential of CO: Negative Bond-ends and Positive Bond-cylindrical-surface | Scientific Reports

bond - How can the dipole moment of carbon monoxide be rationalised by molecular orbital theory? - Chemistry Stack Exchange
Chemsir on X: "Do you notice that there is a dative covalent bond in a carbon monoxide molecule?🤔#chem #chemistry #science #CarbonMonoxide #CO #ElectronDiagrams #ElectronDiagram #DotAndCrossDiagram #octet #CovalentBond #DativeCovalentBond #hkdse #dse ...

Carbon Monoxide, CO Molecule. Сarbon and Oxygen Atoms are Connected by a Triple Bond Stock Vector - Illustration of pollution, structural: 239578195
Carbon monoxide, CO molecule. Сarbon and oxygen atoms are connected by a triple bond that consists of two pi bonds and one sigma bond. Vector illustra Stock Vector Image & Art -




![The bond length of `C-O` bond in carbon monoxide is `1.128A` The `C-O` bond in `[fe(CO)_(5)]` is . The bond length of `C-O` bond in carbon monoxide is `1.128A` The `C-O` bond in `[fe(CO)_(5)]` is .](https://i.ytimg.com/vi/nP3IBxchKGA/maxresdefault.jpg)