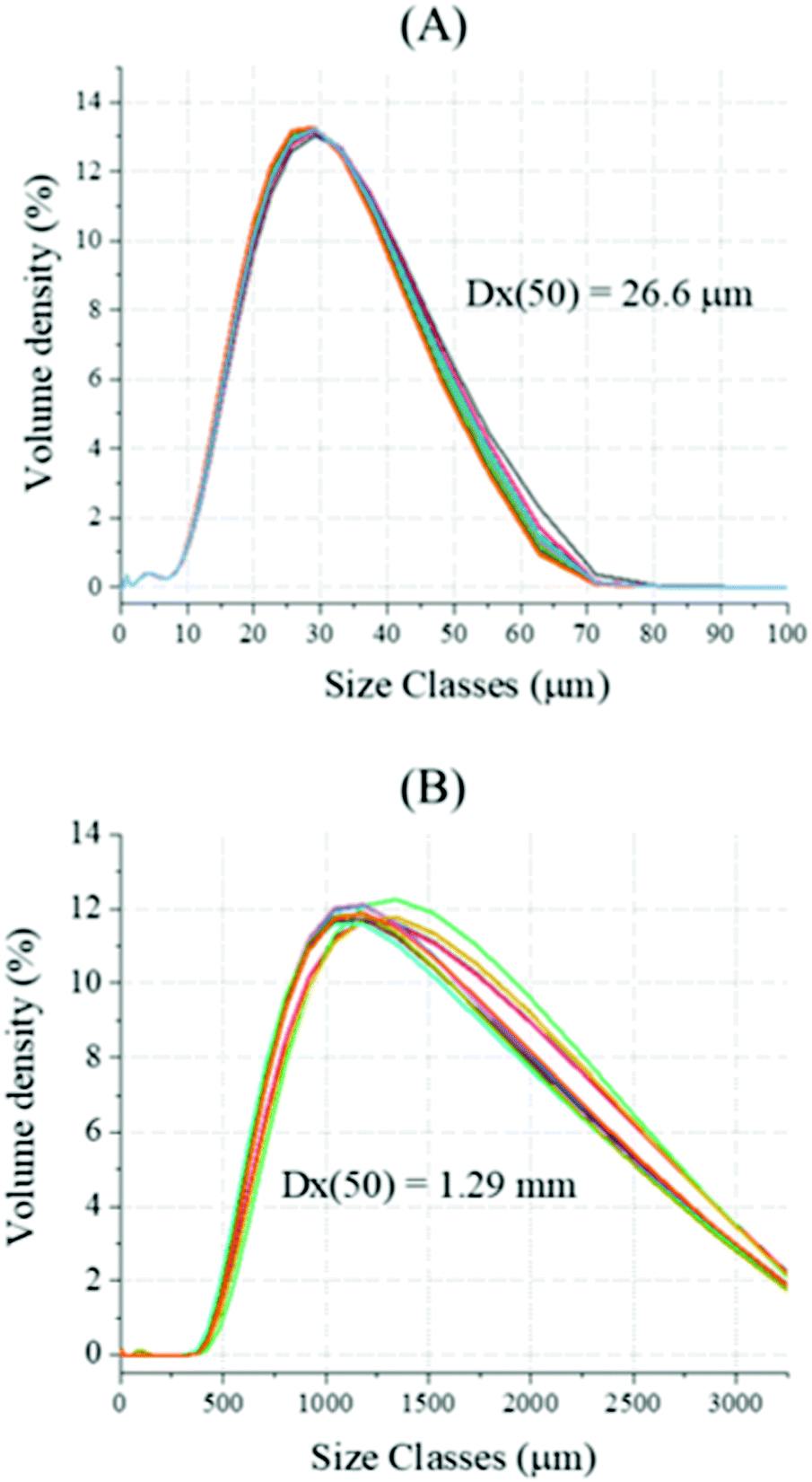
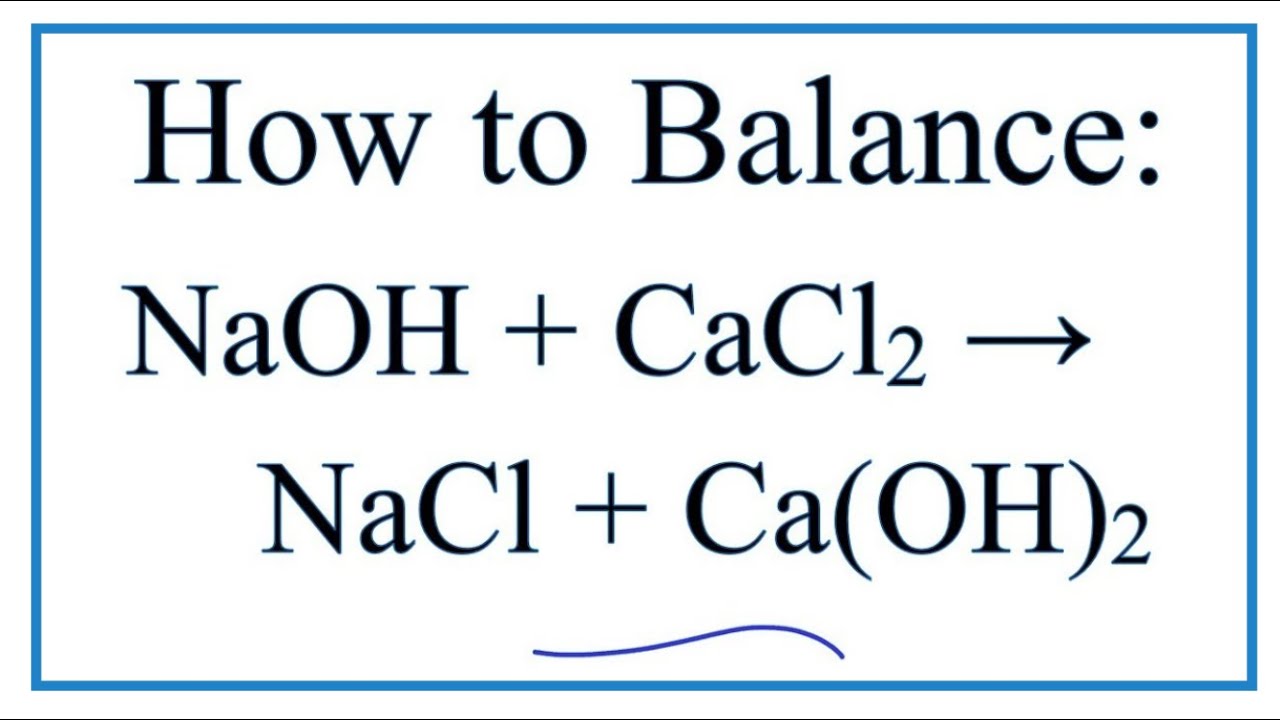
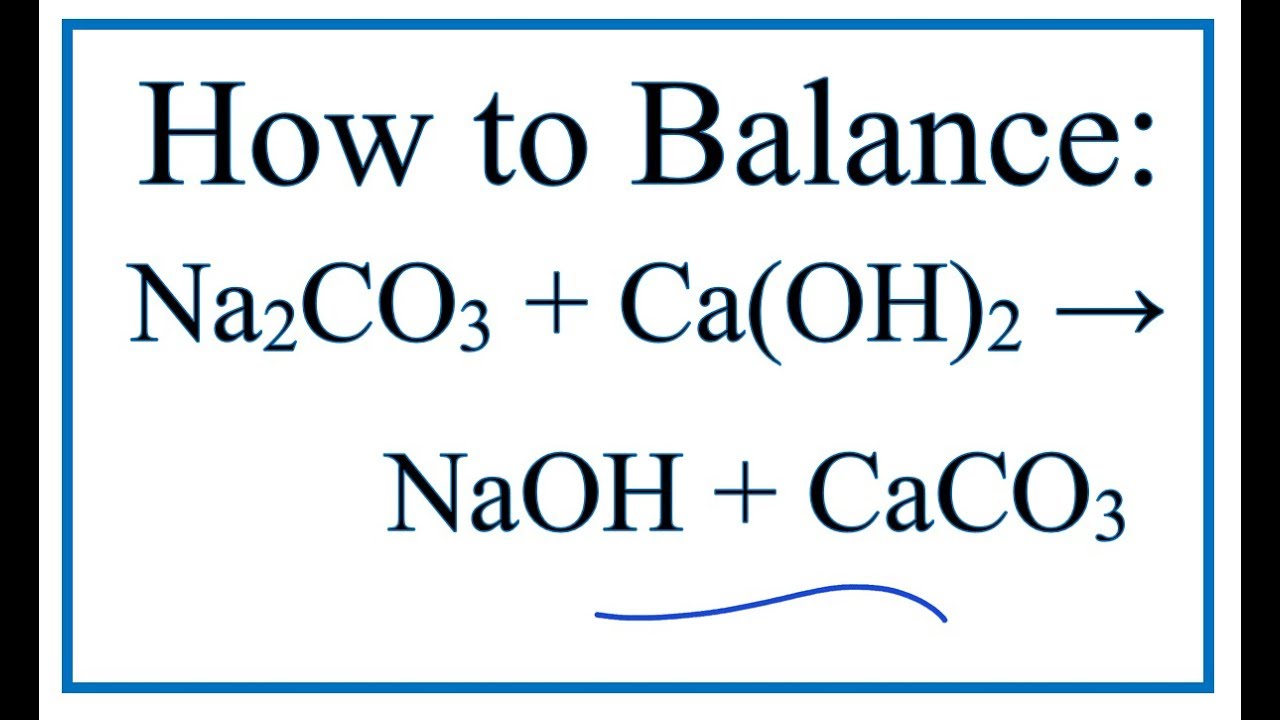
SOLVED: If 3.5 moles of sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2CO3 + Ca(OH)2 NaOH + CaCO3

Figure 3 from Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing

Bases. Jars containing calcium carbonate (Ca2CO3), copper oxide (CuO) and sodium hydroxide (NaOH). These compounds are classified as bases, because th Stock Photo - Alamy

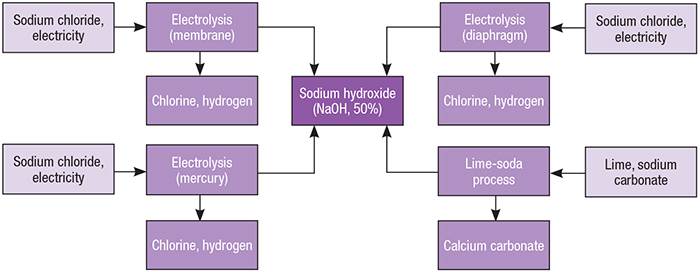
Sodium Hydroxide Production from Lime and Sodium Carbonate | Economic Analysis | by Intratec Solutions | Intratec Products Blog | Medium
![PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/00e03ec2cffecd61e840107d0d9fcb645c1e1c00/3-Figure1-1.png)
PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar

The Effect of Variation Concentration Sodium Hydroxide (NaOH) on the Structure of Calcium Carbonate (CaCO3) Based on Natural Sand | Scientific.Net

Sodium hydroxide and sodium silicate properties which was used in this... | Download Scientific Diagram
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio
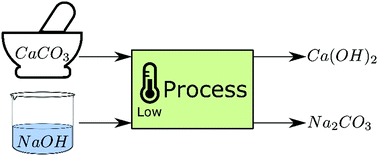
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)
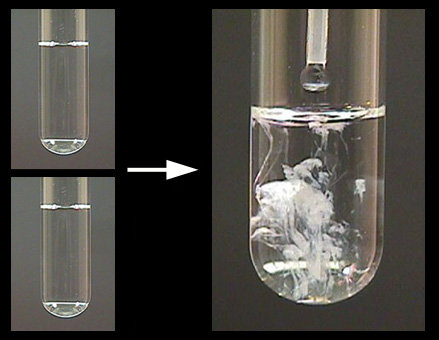
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through productio