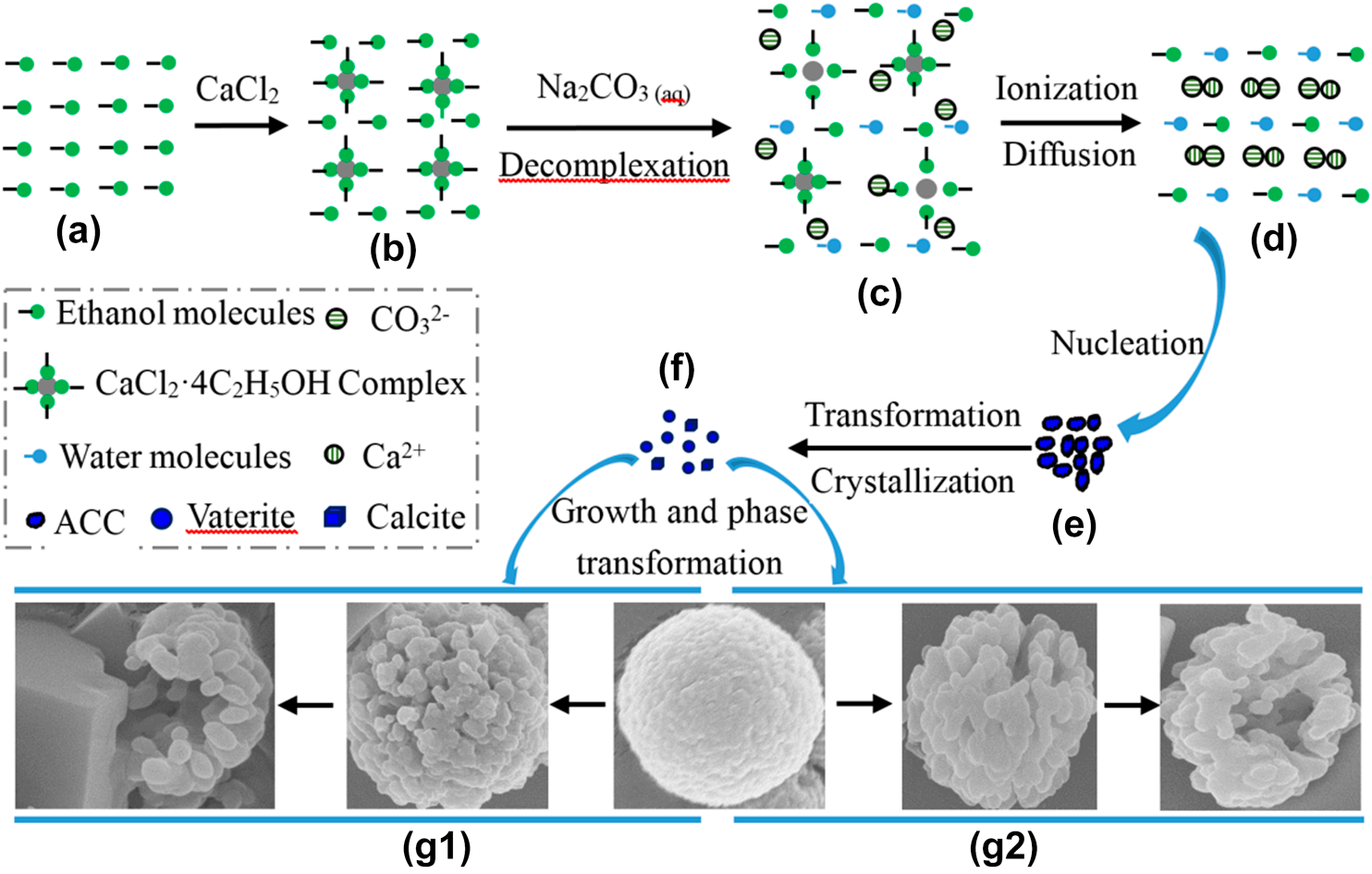
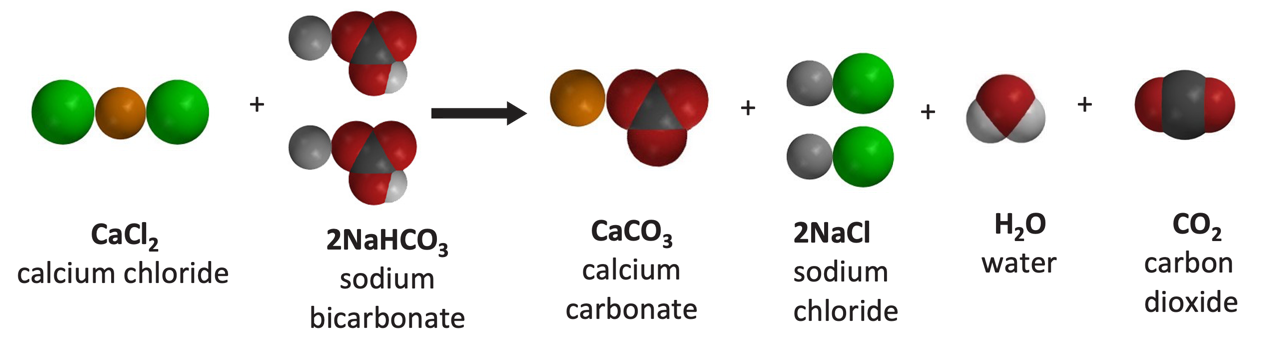
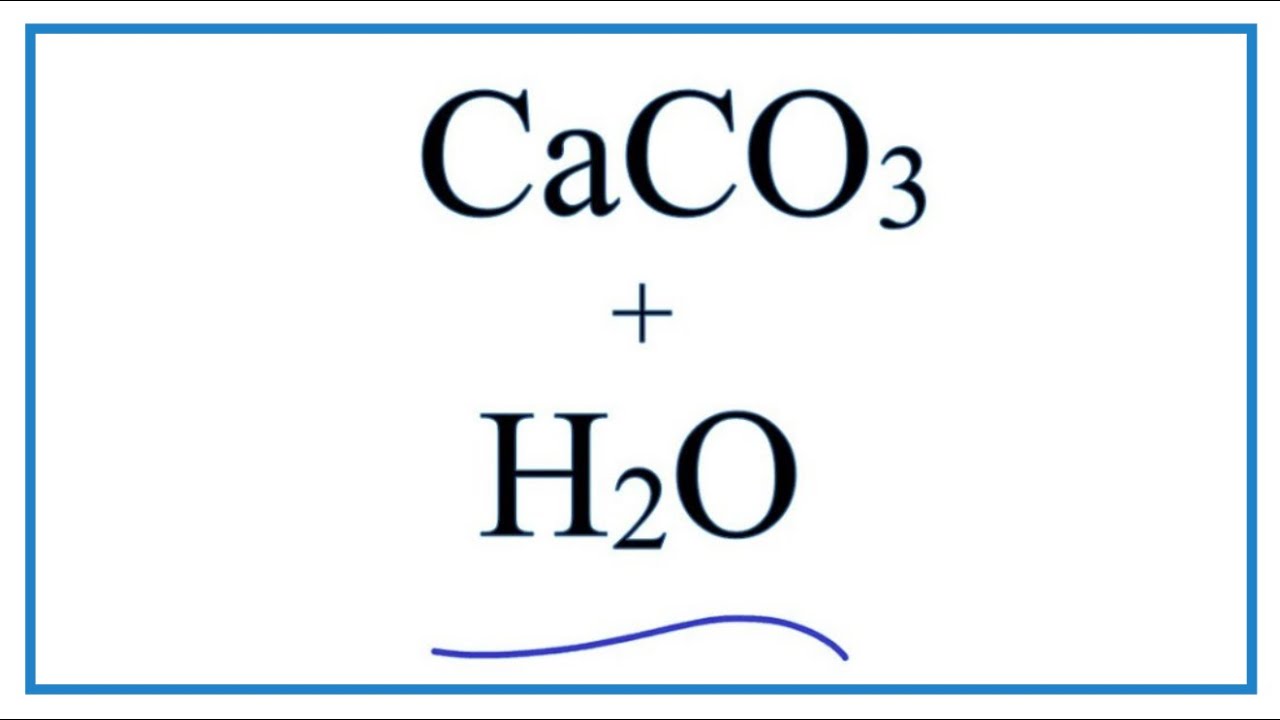
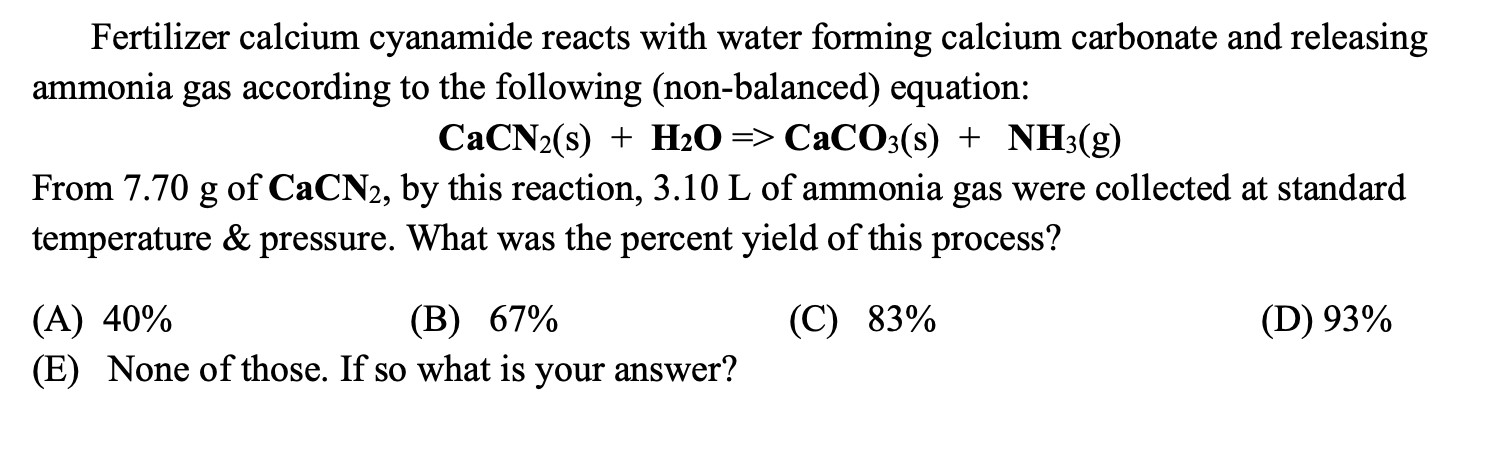Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

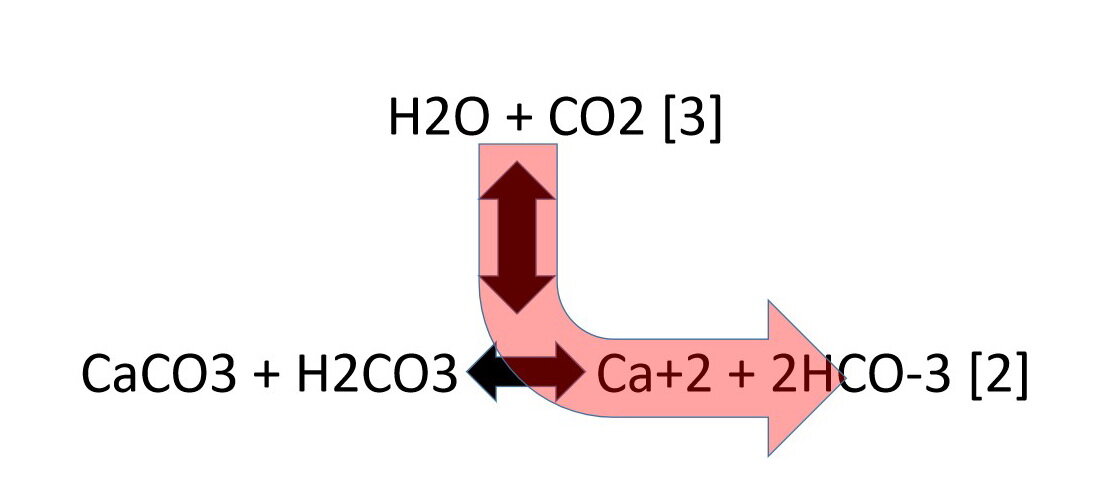
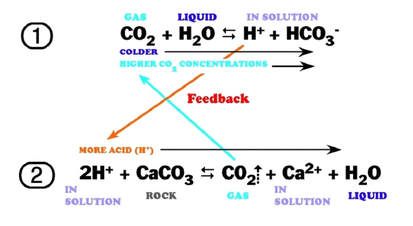
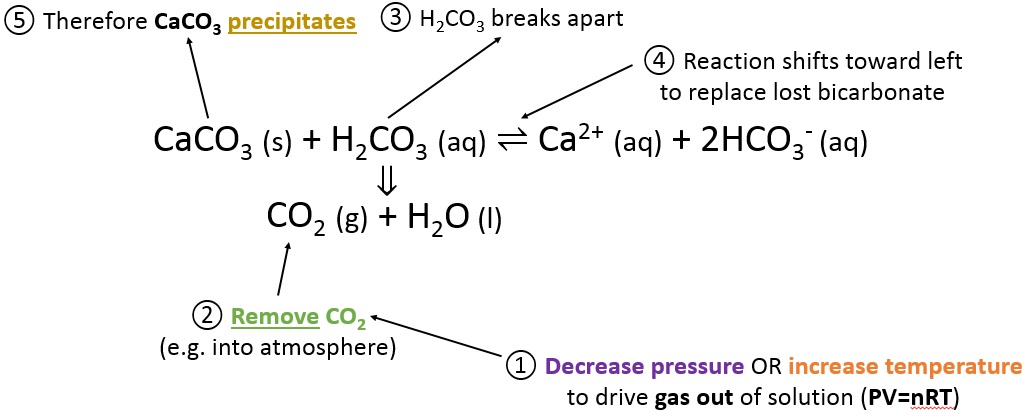
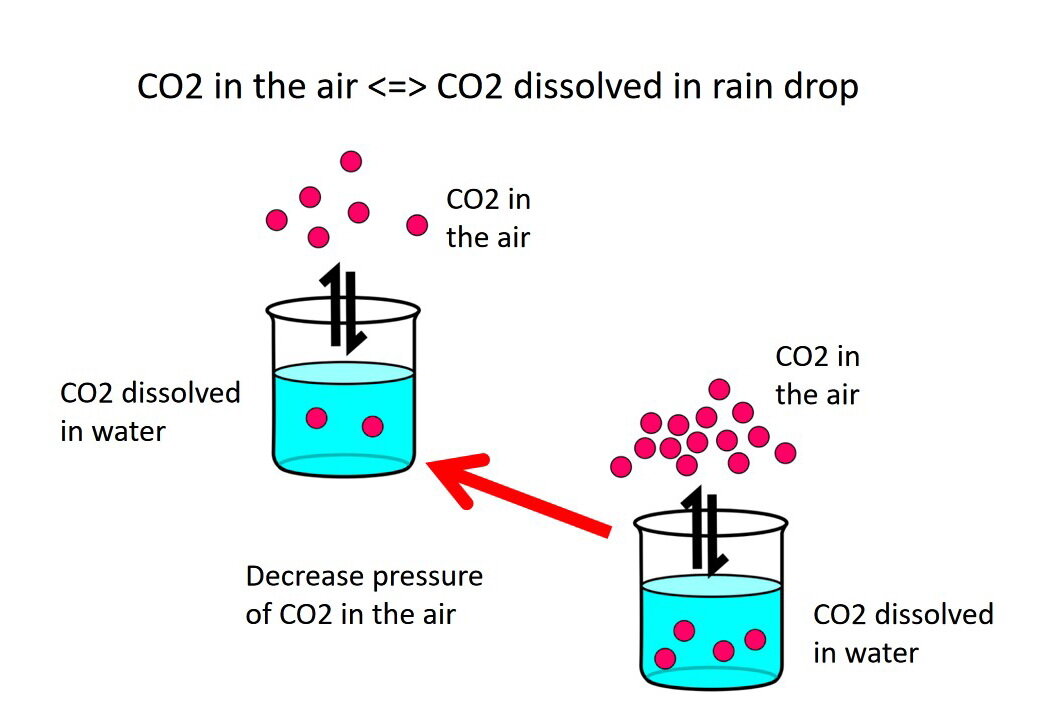
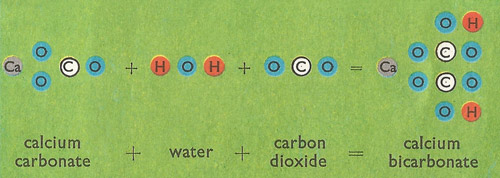
Summary of the reactions between carbon dioxide (CO2) with water (H2O)... | Download Scientific Diagram

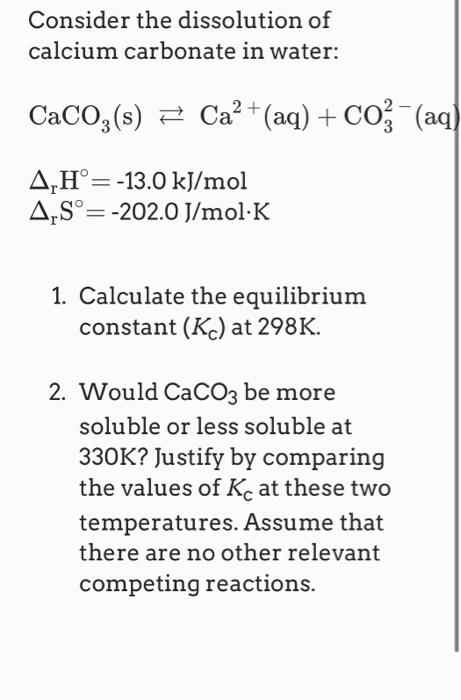
One method of determining the proportion of calcium carbonate in a coral is to dissolve a known mass of the coral in excess acid and measure the volume of carbon dioxide formed.
Calcium carbonate reacts with hydrochloric acid to produce calcium chloride, water, and carbon dioxide gas. What is the balanced equation for it? - Quora

Why lime water turns milky due to formation of white precipitate of calcium carbonate , when carbon dioxide gas is passed through lime water ?