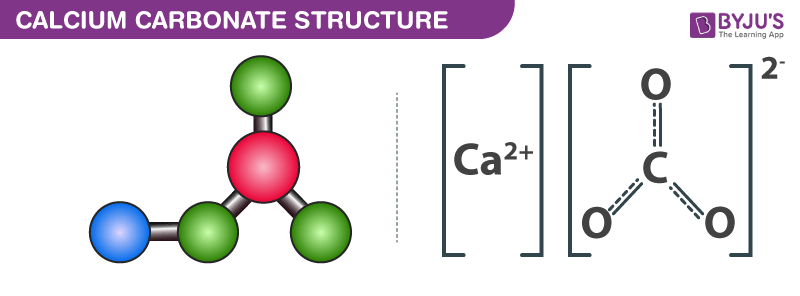
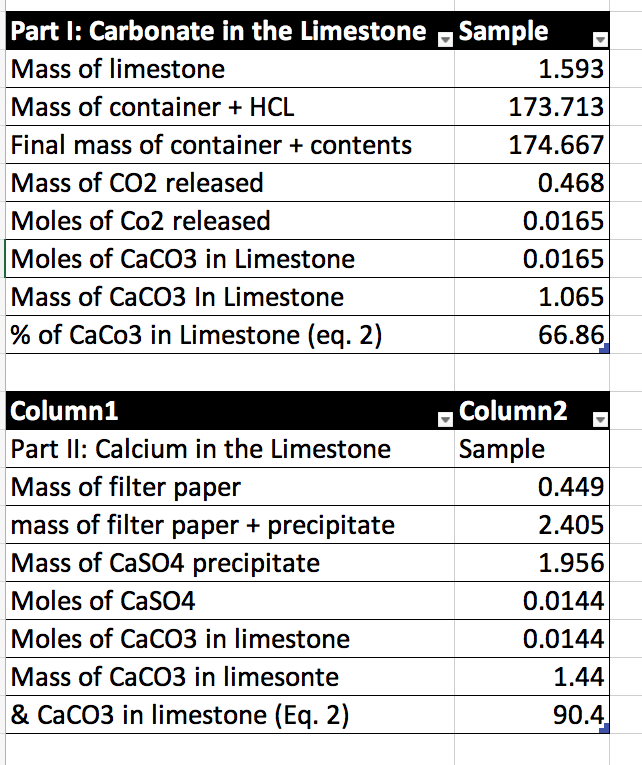
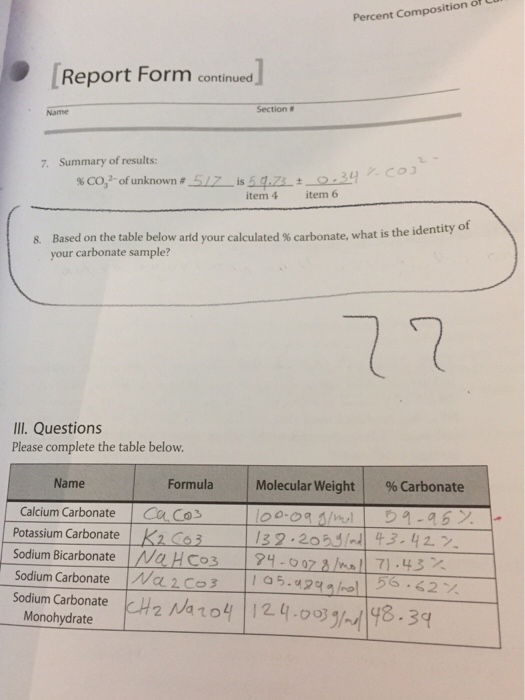
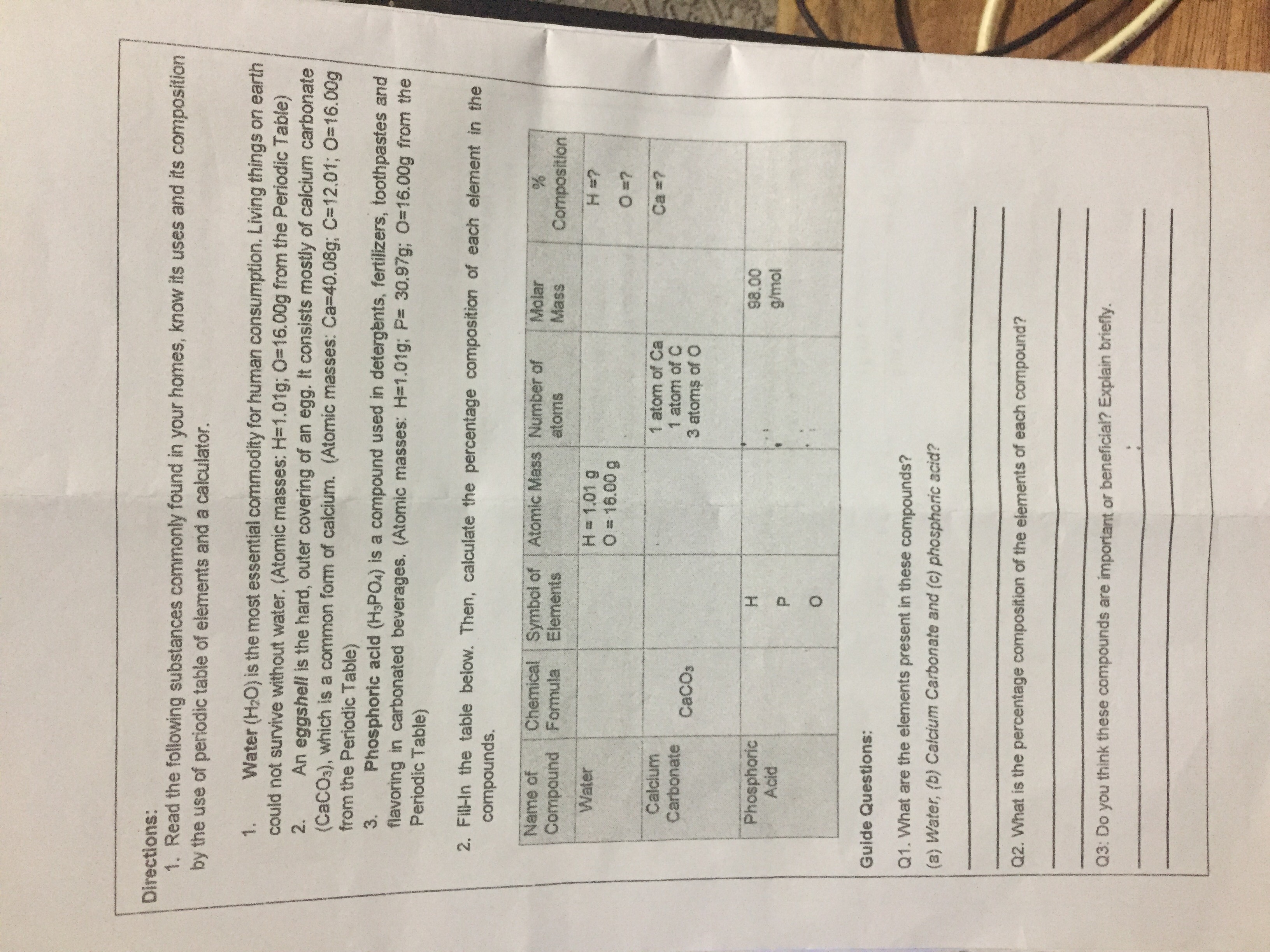
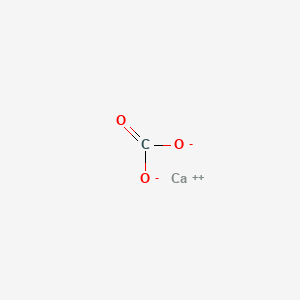
The percentage of three elements calcium, carbon and oxygen in a sample of calcium carbonate is given as: Calcium = 40%; Carbon = 12.0%; Oxygen = 48% If the law of constant

Mineralogical composition of limestone and mussel shells showing the... | Download Scientific Diagram

Mean calcium carbonate content in texturally different soils at 0-15... | Download Scientific Diagram
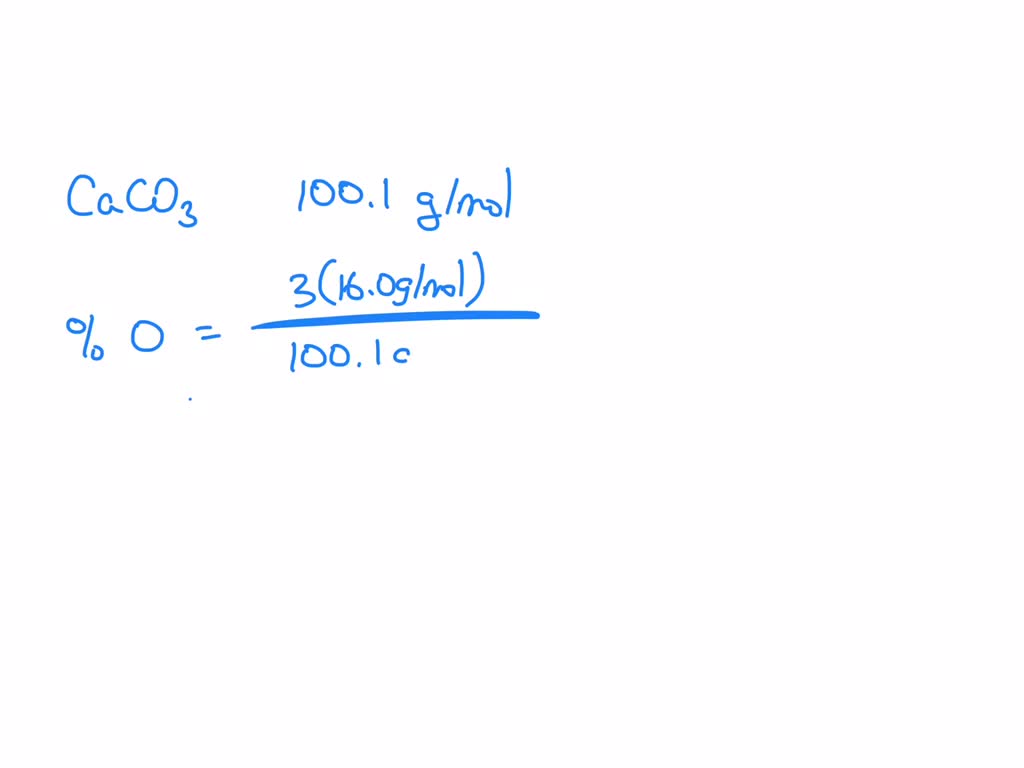
SOLVED: The formula of calcium carbonate Is CaCO3 Determine the percent composition of oxygen in this compound: Round answer t0 three significant figures: Provide your answer below:
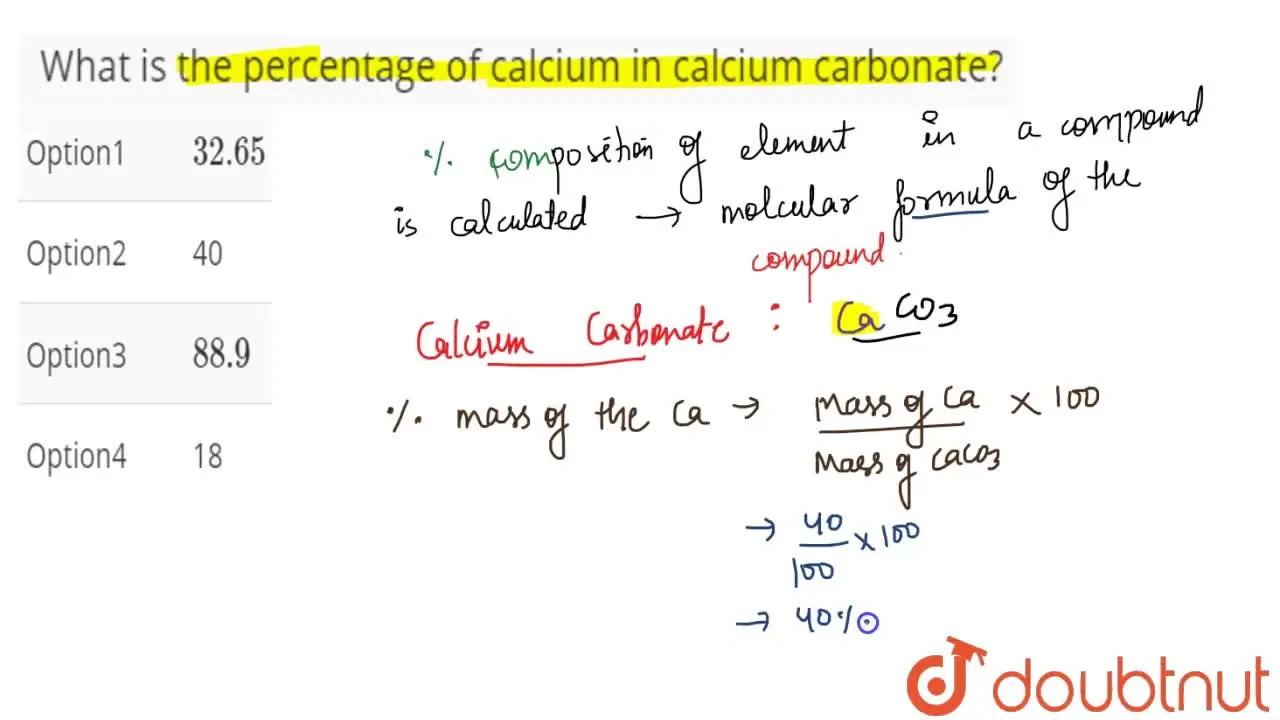
Calculate the % of each element in calcium carbonate. (Atomic mass: C – 12, O – 16, Ca – 40) - Sarthaks eConnect | Largest Online Education Community

The percentage of 3 element are as follows in calcium carbonate (CaCO_{3}) Calcium 40%, carbon 12%, Oxygen 48%. law of constant proportion is true what weight of these element will be present