Table of atoms in molecules, chemical formula of carbon,oxygen,hydrogen and nitrogenmolecules.Educational and study content of chemistry and science s Stock Vector Image & Art - Alamy

SOLVED: A compound contains, by mass, 48.64% carbon, 8.16% hydrogen and 43.209 oxygen The empirical formula of this compound is: CzHsOz CaHgO3 CzH;O CzH;O

A compound of carbon, hydrogen, and nitrogen contains the three elements in the respective ratio... - YouTube

An organic compound contains 20 % carbon, 6.7 % hydrogen, and 46.67 % nitrogen. Its molecular weight was found to be 60 . Find the molecular formula of the compound.

The empirical formula of an organic compound containing carbon and hydrogen is CH2 . The mass of one litre of this organic gas is exactly equal to that of one litre of
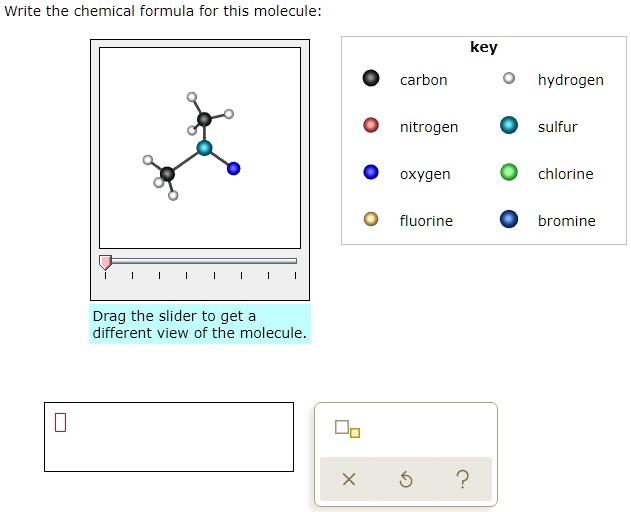
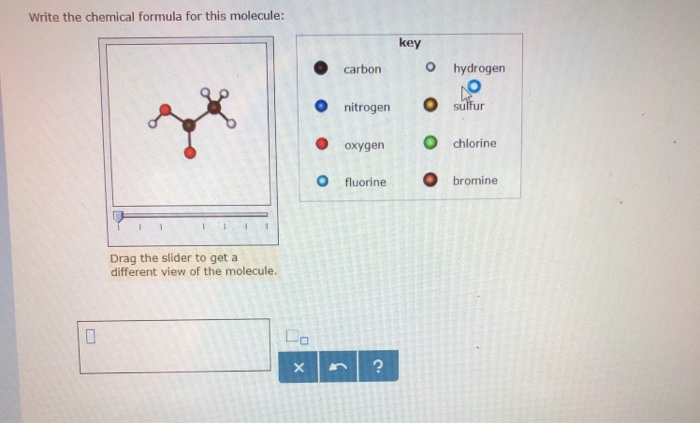
SOLVED: Write the chemica formula for this molecule: key carbon hydrogen nitrogen sulfur oxygen chlorine fluorine bromine Drag the slider to get different view of the molecule

Calculate the Empirical Formula for a compound with the following composition: 46.16% carbon; 53.84% nitrogen 1)Change % to grams (if needed) 2)Convert. - ppt download
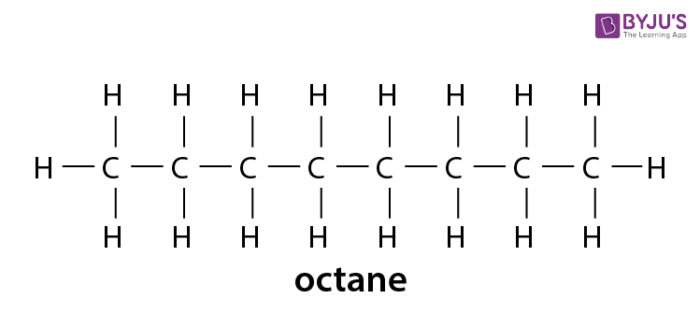
Alkanes - Formula, Definition, Structure, Properties, List of Alkanes, Videos, Examples and FAQS of Alkanes.