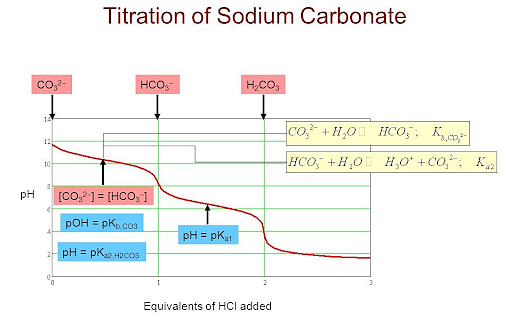
experimental chemistry - Equivalence point of titration of Sodium carbonate - Chemistry Stack Exchange

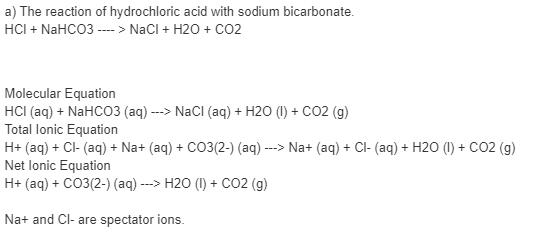
Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube
Reactions and temperature changes - Exothermic and endothermic reactions - AQA - GCSE Chemistry (Single Science) Revision - AQA - BBC Bitesize

We can measure the concentration of HCl solution by its reaction with pure sodium carbonate. 2 H+ + Na2CO3 ? 2 Na+ + H2O + CO2 Complete reaction with 0.9639 0.0005 g of Na2CO3 required 28.20 0. | Homework.Study.com
What is the reaction between baking soda and hydrochloric acid? How can this reaction be used practically? - Quora
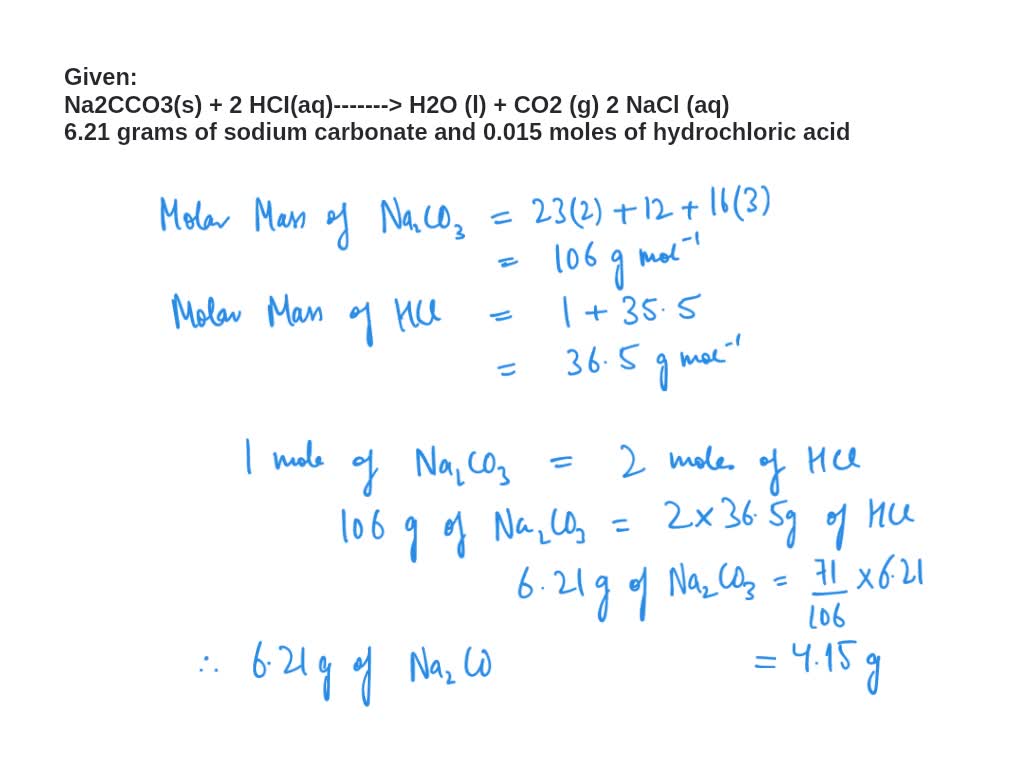
SOLVED: Use this balanced equation to solve the following problem: Na2CO3 (s) + 2 HCl (aq) â†' H2O (l) + CO2 (g) + 2 NaCl (aq). 6.21 grams of sodium carbonate are

Dilute hydrochloric acid was added to sodium carbonate added to calcium ions. Write the equation for the - Brainly.in